
The polarity of a bond depends on the electronegativities of the bonded atoms.
A polar molecule arises when one of the atoms exerts a stronger attractive force on the electrons in the bond. (b) The dipole is represented by an arrow with a cross at the tail. Symbols + and indicate the polarity of the HCl bond. When atoms come together in chemical bonding, they share electrons. (a) Unequal sharing of the bonding pair of electrons between H and Cl leads to partial positive charge on the H atom and partial negative charge on the Cl. Arranging the electrons as lone pairs to form bonds with each atom. Bonds between carbon and other elements such as oxygen and nitrogen are polar. In chemistry, polarity refers to the way in which atoms bond with each other. Locating the central atom of the molecule. Polar covalent bonds are very common, because the electronegativities of the two atoms at either end of the bond are very unlikely to be the same unless both.

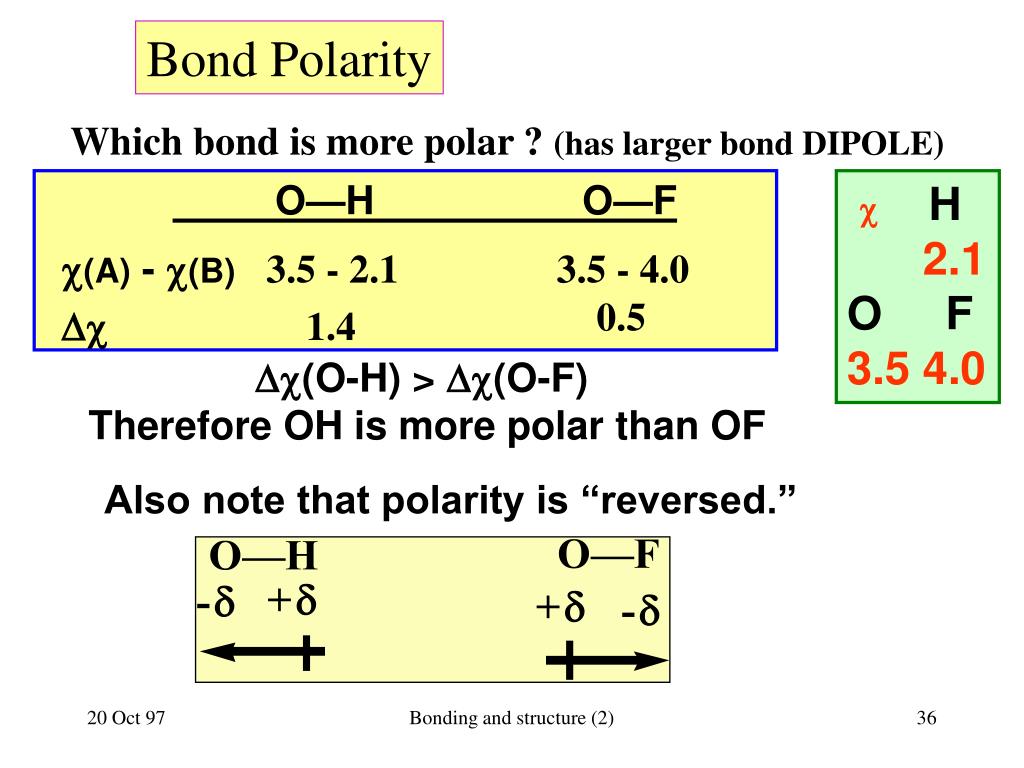
The more electronegative atom gains partial negative charge and other atom gets a partial positive charge. These atoms unequally share the bonded pair of electrons. This indicates how strong in your memory this concept is. (Credit: Jodi So Source: CK-12 Foundation License: CC BY-NC 3. Counting the total number of valence electrons of the molecule. Learners are shown how the difference in electronegativity between two bonded nuclei determines the degree of polarity of the bond between them. The covalent bond formed between two atoms is said to be polar if the electronegativity of both atoms is not equal. Covalent compounds may have polar or nonpolar bonds, depending on their arrangement of atoms. Molecules with polar covalent bonds have a positive and negative side.

Molecular polarity depends on both individual bond polarities and molecular geometry, the latter of which we can predict using VSEPR theory. Covalent bonds can be non-polar or polar and react to electrostatic charges. The dipole moment of the chemical bond is used to calculate the bond polarity. \): As the electronegativity difference increases between two atoms, the bond becomes more ionic.\): A nonpolar covalent bond is one in which the distribution of electron density between the two atoms is equal. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. The polarity of bonds mainly arises from the act between molecules and atoms with various electronegativities. The separation of a charge within a molecule is referred to as bond polarity.


 0 kommentar(er)
0 kommentar(er)
